Quality management¶
- Contenu
- Quality management
Control points¶
« Control points » gather controls to be carried on.
Example : a microbiological control at the reception of a raw material can include :
- Salmonella quantity control ;
- Coagulase Staphylococcus + quantity control.
To create a control point, go to Repository> Quality> Specifications> Control points. Press « Create » and choose « Folder ».
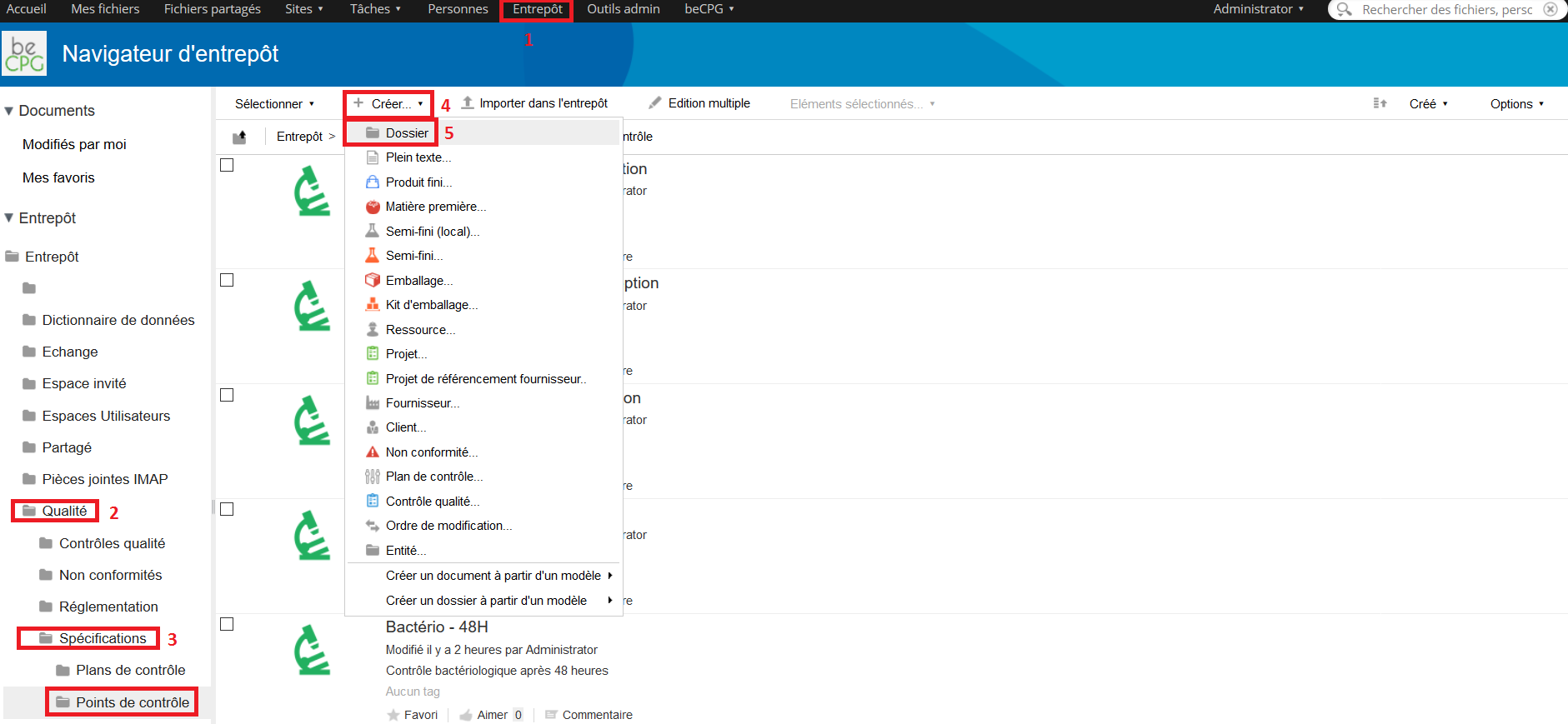
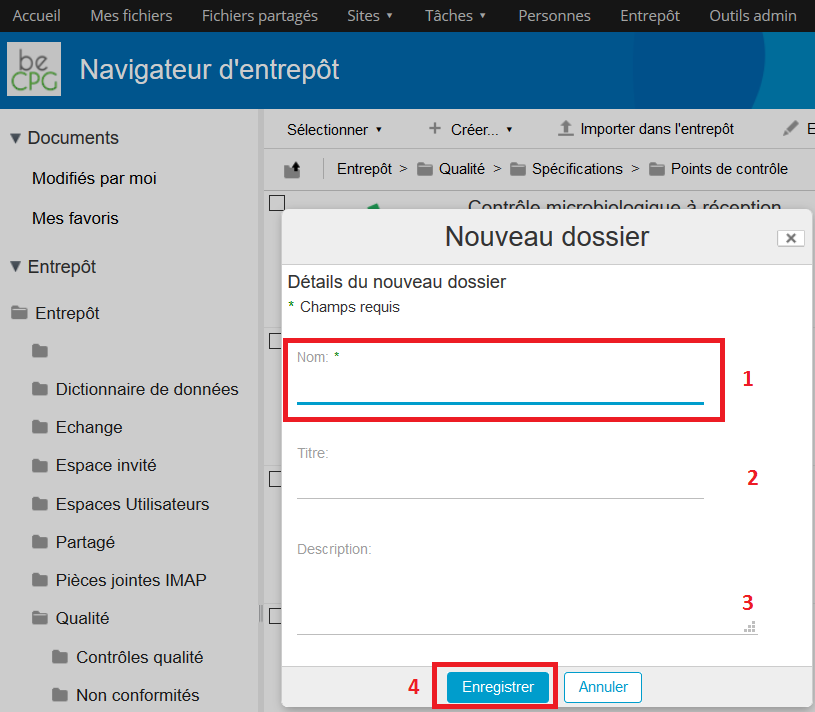
A form appears allowing to fill the control point’s name. If this latter has to be spelled out, the « Description » field can be filled.
To fill the different actions to be carried on in this control point, go to the « Controls » list and press « Add ».
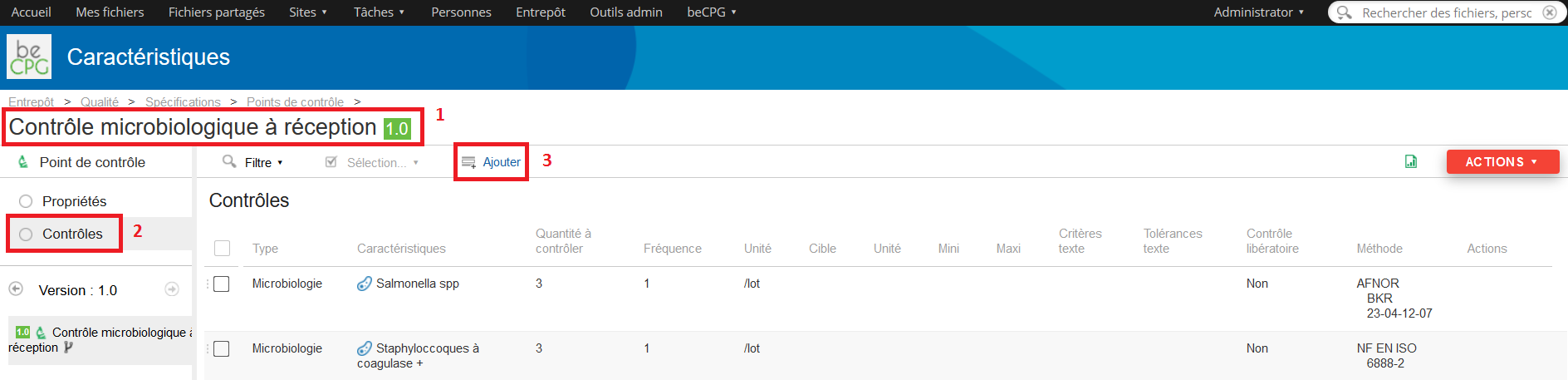
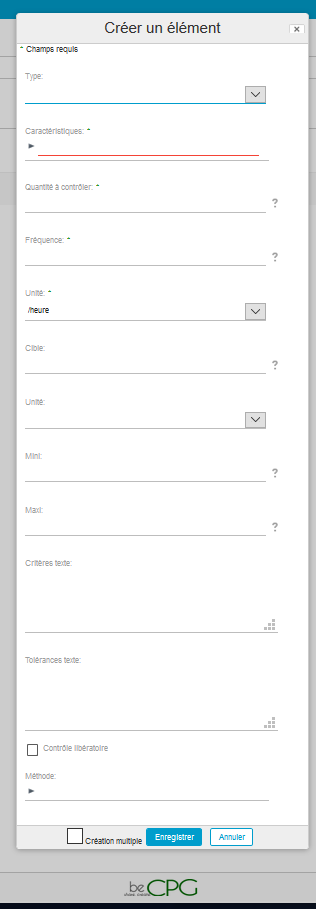
A form will appear and allows you to mention:
- The control type (microbiologic, visual, physicochemical, etc.) ;
- The characteristic to be checked (bacteria, colour, pH, etc.) ;
- The quantity to be controlled (number of batches which have to be analysed) ;
- The control frequency ;
- The unit of this frequency ;
- The target or desired value for the characteristic ;
- The unit of this target ;
- The minimum value for this target ;
- The maximum value for this target ;
- A text criteria;
- A text tolerance;
- If it is a withholding control or not ;
- The control method which has to be used.
Note : the methods list can be filled in the admin part of beCPG (beCPG> Administration> beCPG> Quality list> Control method).
Once the control points have been created, they can be used in the control plans.
Control plans¶
Control plans allow you to define the whole prelevements to be done in order to carry on actions mentioned in the control points.
Example : when a raw material is received, these controls have to be carried on :- Visual control ;
- Physico-chemical control ;
- Microbiological control ;
- Organoleptic control.
Two possibilities exist to create a control plan :
- Go to the folder where the control plan has to be created and click on « Create » and then « Control plan ».
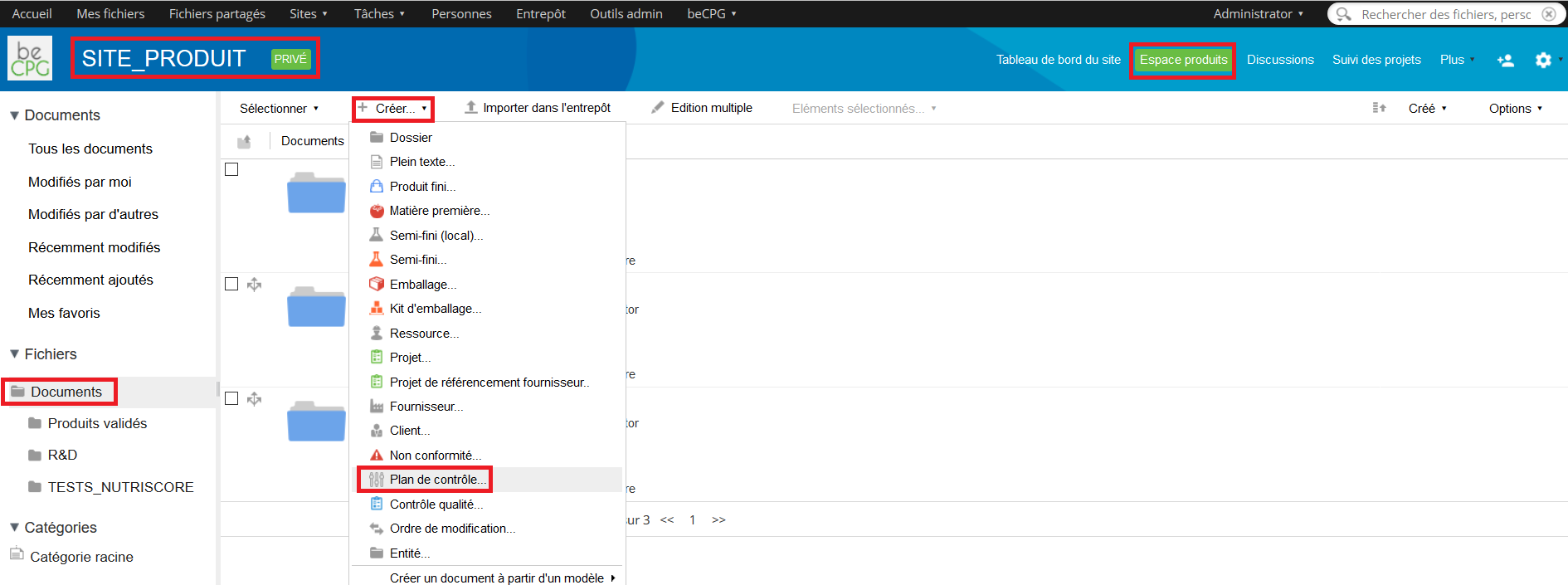
- Go to Repository> Quality> Specifications> Control plans. Press « Create » and choose « Folder ».
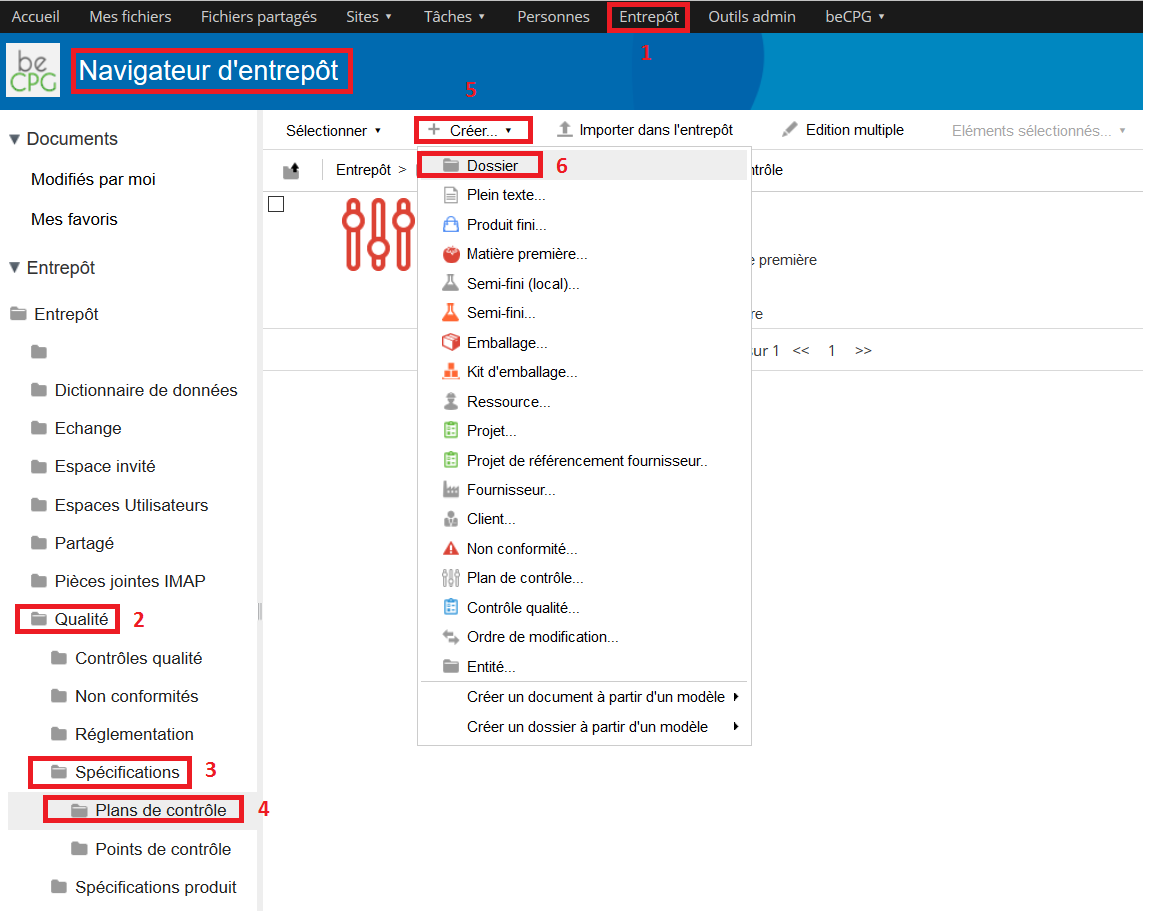
A form appears in order to fill the name of the control plan. If this latter has to be spelled out, the « Description » field can be filled.
To mention the different actions that have to be achieved to carry on this control plan, go to the list « Sampling » and press « Add ».
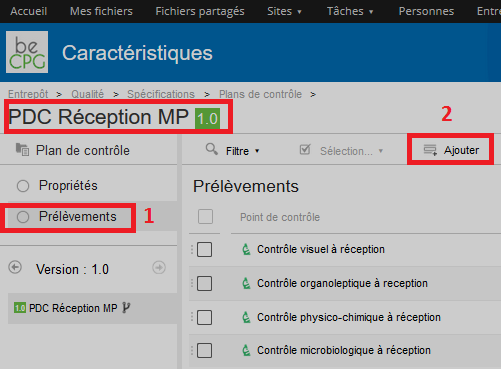
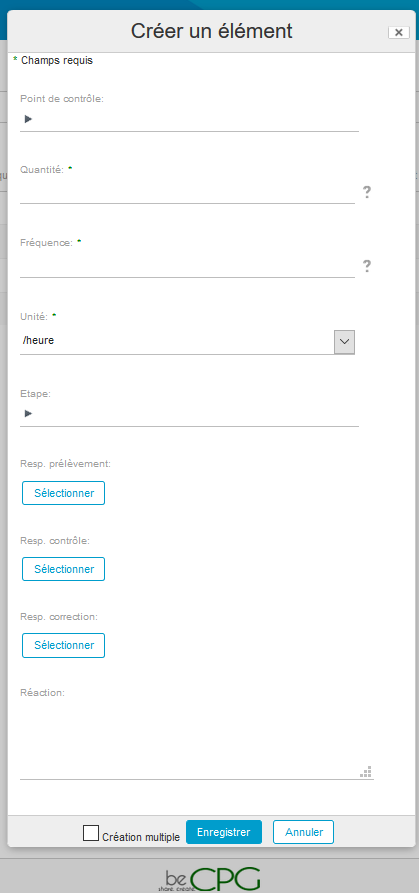
A form will appear and allows you to mention:
- The control point (cf. « Control point » section) ;
- The quantity to be controlled;
- The control frequency ;
- The unit of this frequency ;
- The step where this control has to be carried on. Note : the steps list can be filled in the administration part of beCPG (beCPG> Administration beCPG> Quality list> Control steps).
- The responsible for the sampling ;
- The responsible for the control;
- The responsible for the correction;
- A reaction.
Once the control plans have been created, they can be used in the quality controls.
Quality controls¶
Quality controls allow you to specify the control plans which have to be achieved on the different products of the catalog.
To create a quality control, go to Repository> Quality> Quality controls and press « Create » and then « Folder ».
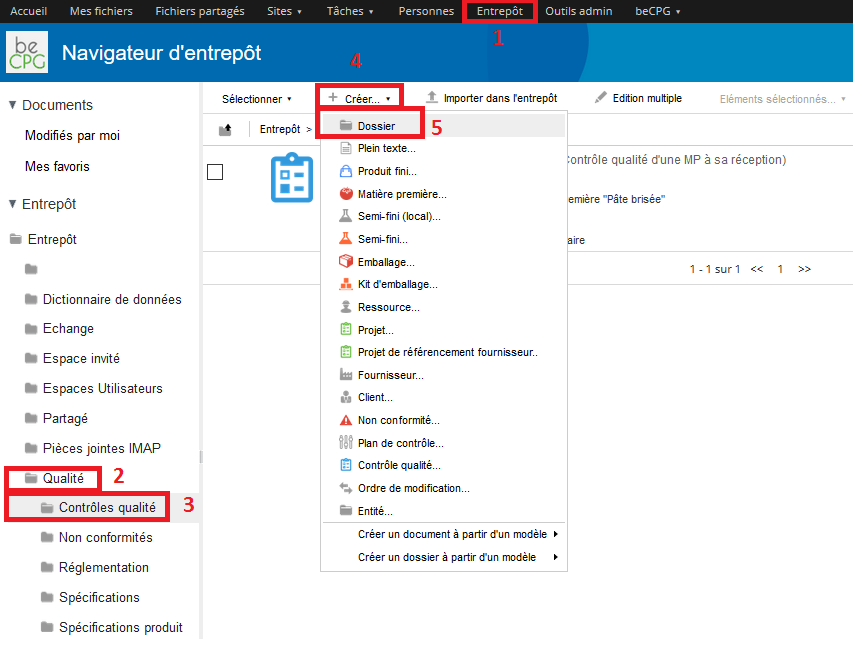
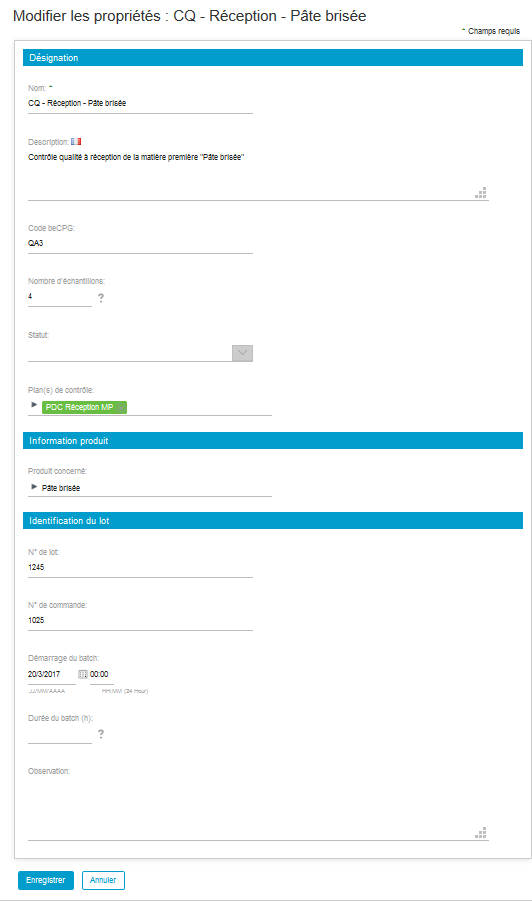
A form appears and allows you to mention the internal name of the quality control. This latter may have a more formal name, in which case, the « Title » field can be used. If the name of the quality control has to be spelled out, the « Description » field can be filled.
- The number of samples to be tested ;
- The status ;
- The control plan ;
- The concerned product ;
- Its batch number ;
- Its order number ;
- The date of the batch beggining ;
- A comment.
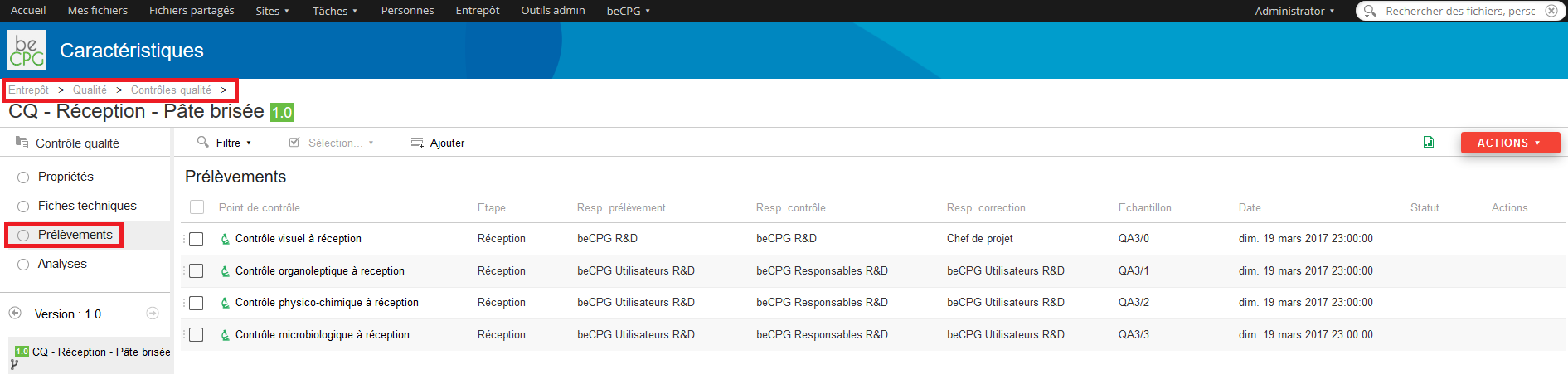
Go to the « Sampling » list to note that the whole samplings mentioned in the control plan have found through to the quality control.
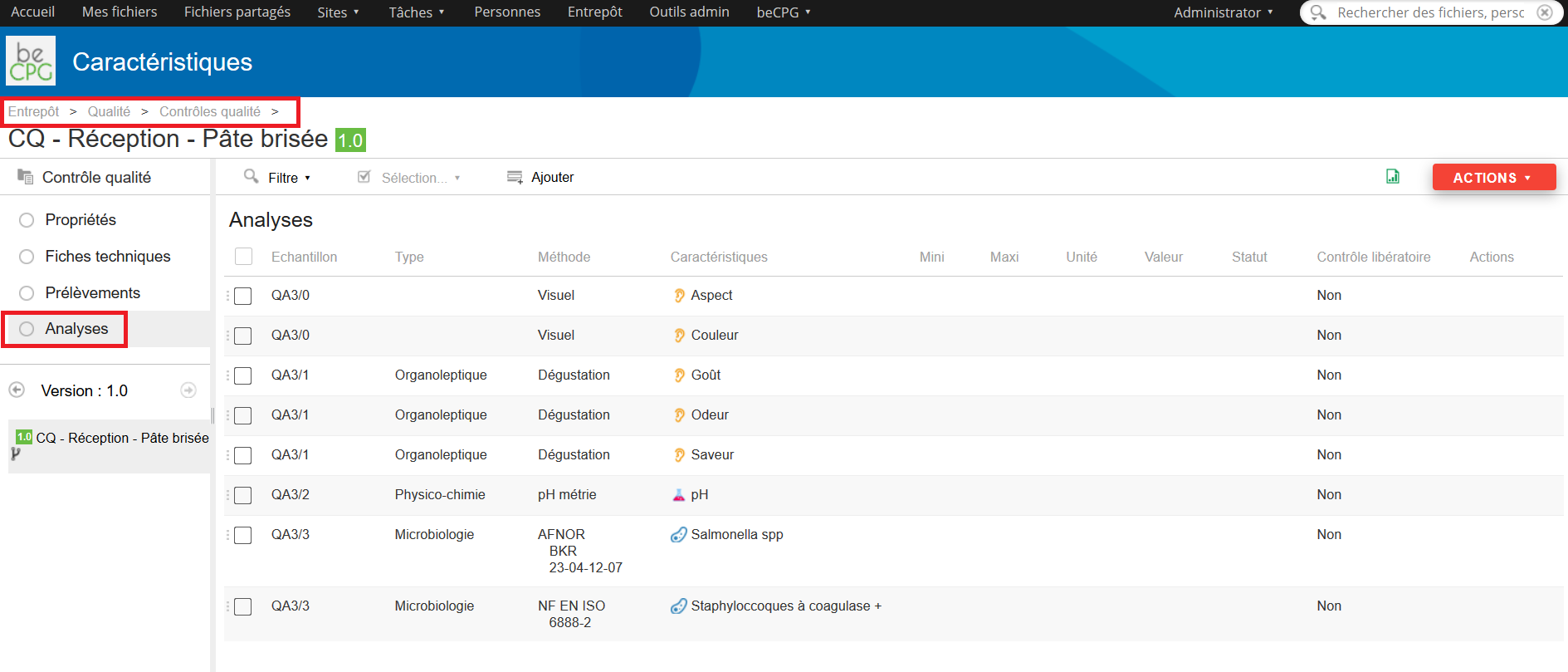
Press the « Analysis » list to see the whole analysis to achieve.
Press the « Technical sheets » list to generate the report analysis of the product once everything is finished.
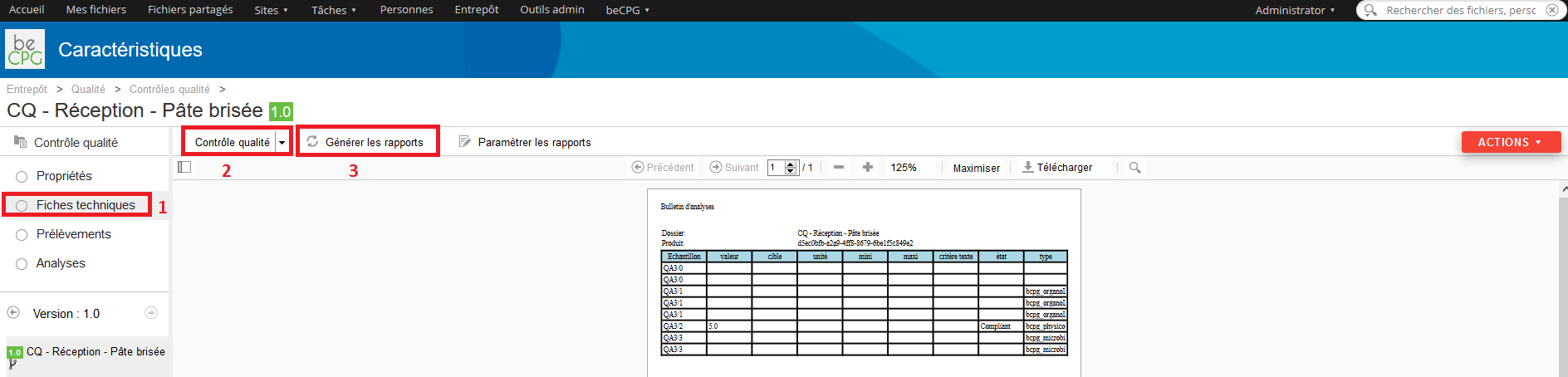
Product specifications¶
Labeling rules¶
Before reading this section, please refer to the labeling documentation.
In order to avoid creating rules specific for each product or each product template, it’s possible to define labeling rules, valid in every cases.
To define labeling rules, go to Repository> Quality> Product specifications. Click on « Create » and then, select « Folder ».
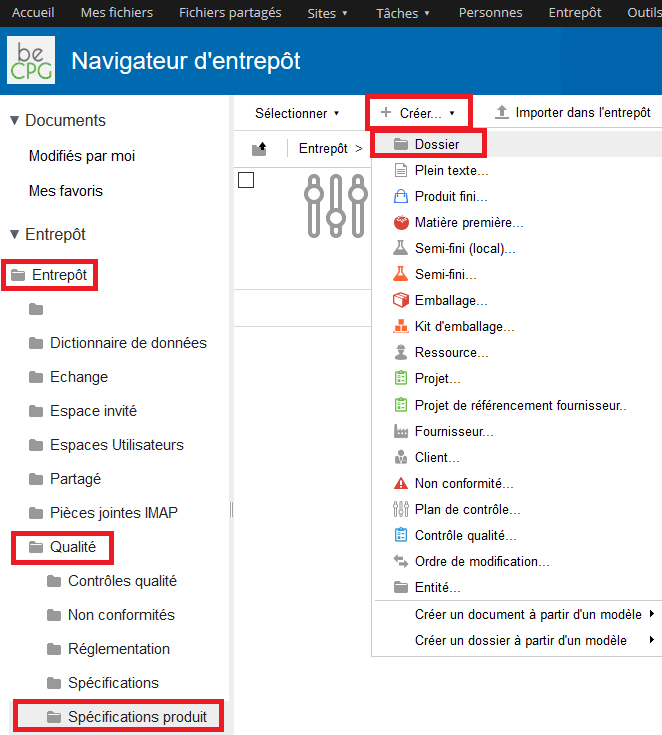
- Its internal name ;
- Its legal name ;
- Its expression, which means its formula ;
- Components concerned by this rule ;
- Language ;
- If the rule is active or not ;
- If the calculation is manual or not.
Note : to fill the form properly, refer to the labeling documentation. Press « Save » to save this new rule.
Thus, when creating a new product and generating its labeling, this rule will be applied automatically (if the entity concerned by the rule is part of the product recipe).
Forbidden ingredients¶
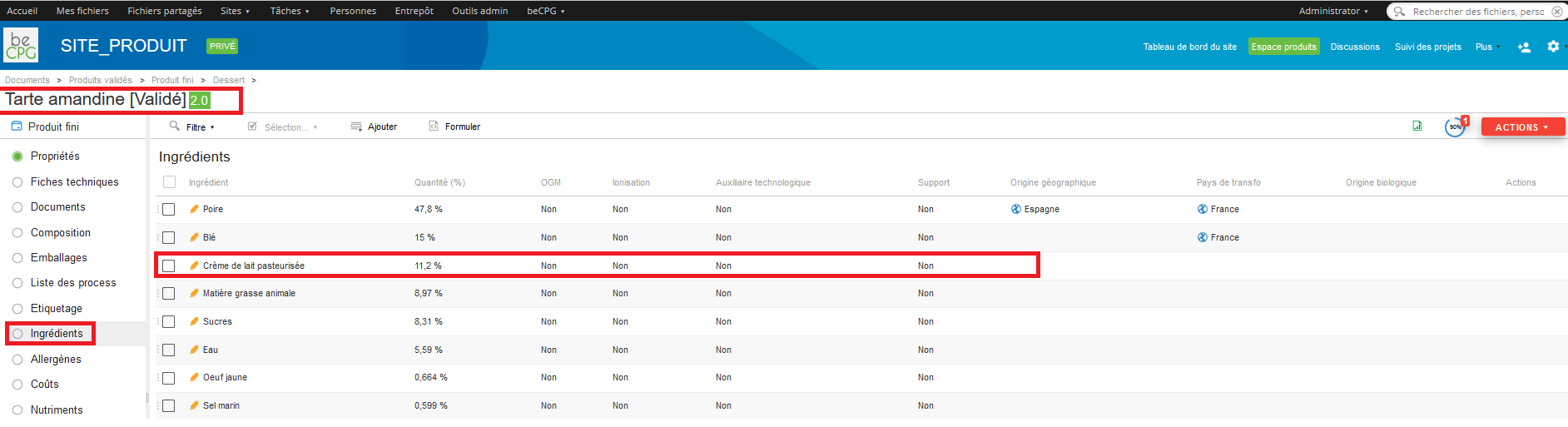
With beCPG, it’s possible to declare forbidden ingredients so that if they are used, an alert is raised.
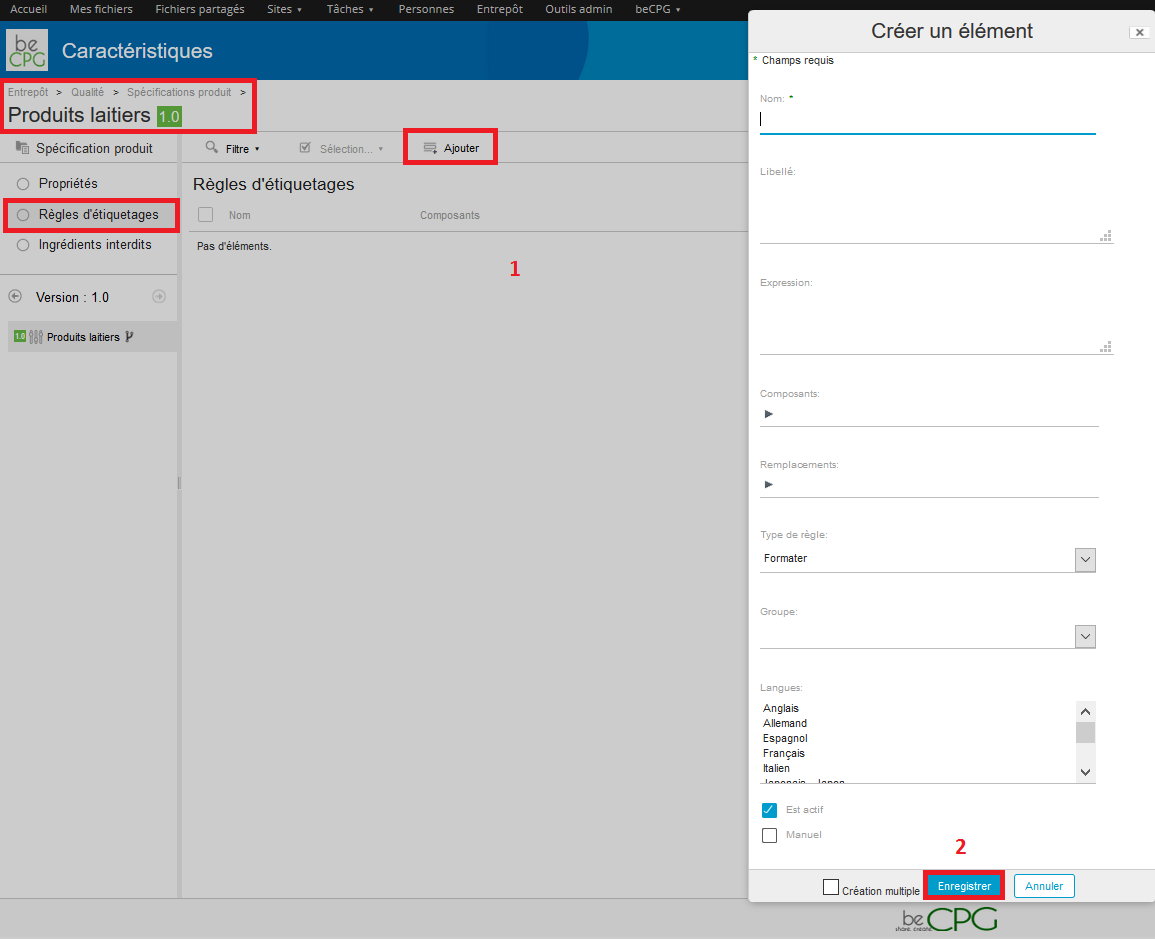
To define forbidden ingredients, go to Repository> Quality> Product specifications. Press « Create » and select « Folder ».

- The requirement level : if the ingredient is authorized, forbidden or if it’s a simple information related to the product ;
- Text area ;
- Maximum value : maximum threshold authorized for the ingredient ;
- Forbidden GMO : if the GMO’s are forbidden or not ;
- Forbidden ionization: if the ionization is forbidden or not ;
- Concerned ingredients : many can be seleted ;
- Geographical origins : it can be forbidden to use certain ingredients from certain countries ;
- Required geographical origins ;
- Biological origins : certain ingredients from certain raw materials can be forbidden.
Press « Save » to save the form.
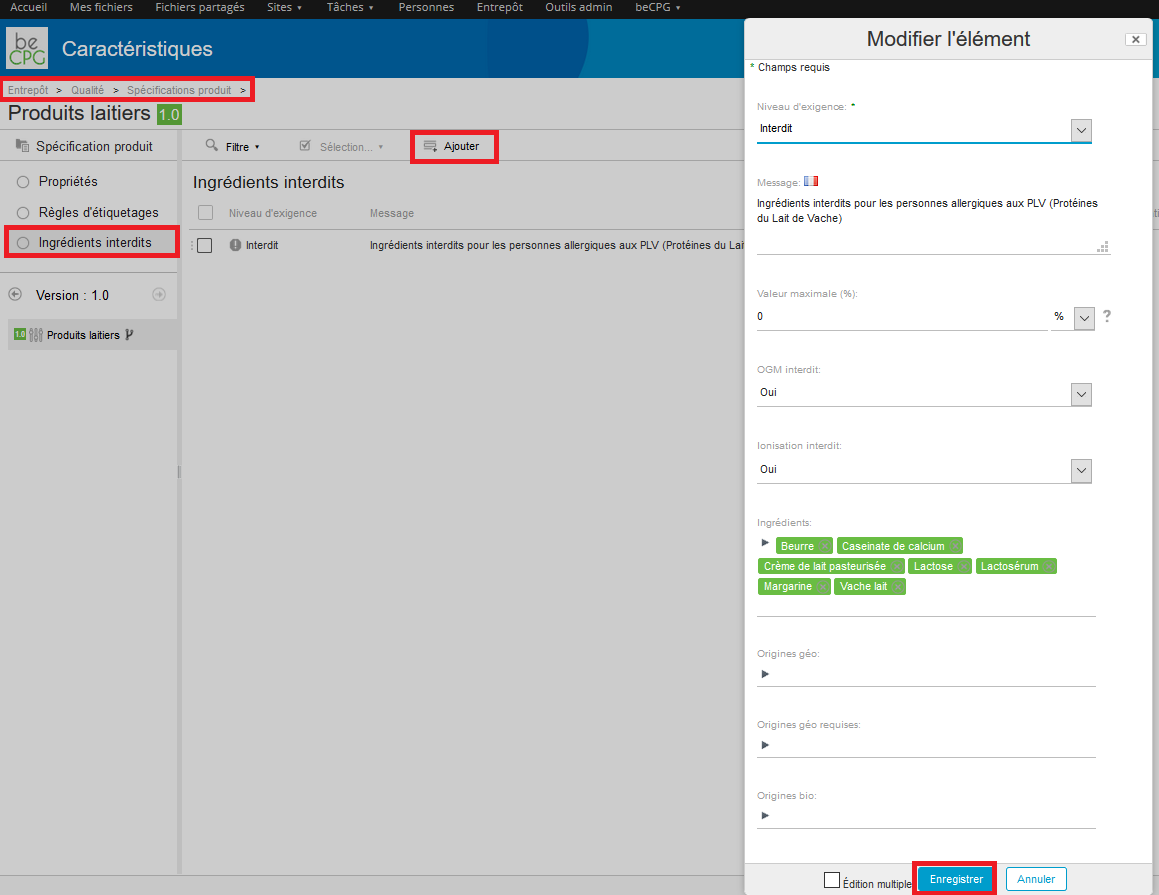
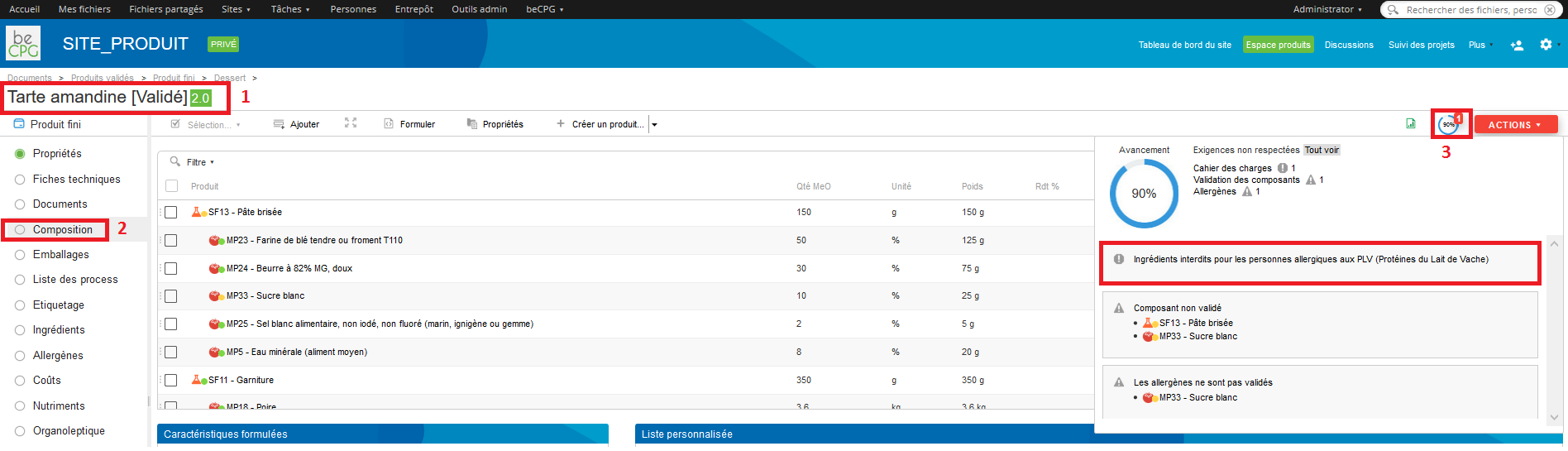
In order to use this specification, go to the product properties (Properties> Edit properties) and then to Formulation> Product specification and choose the specification by clicking on the dropdown list.
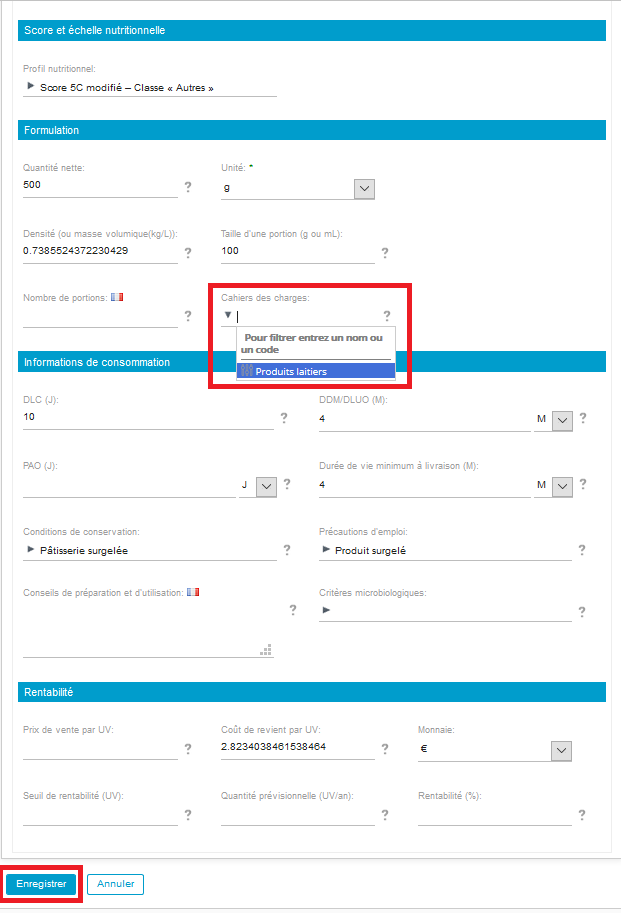
If an ingredient, specified as « Forbidden » in the product specification, is added to a product recipe, by going to the product composition and by pressing the advancement logo, an alert will appear.